Here are three stories from researchers supported by Breast Cancer Now, talking about their innovative techniques and how they will help save lives.
At Breast Cancer Now, research is the core of what we do.

The value of research
Think of a scientist studying breast cancer, and you might have an image in your mind of someone peering down at a Petri dish with cells growing in it – a million miles away from a living breathing person dealing with the disease in the real world. So you’d be forgiven for thinking that growing cells in the lab can never really be useful for developing new breast cancer treatments.
However, in recent years, we have seen the development of incredibly sophisticated ways to study breast cancer in the lab which are driving progress in research. Here are three stories from researchers supported by Breast Cancer Now, talking about their innovative techniques and how they will help save lives.
Dr Ed Carter – replicating DCIS

Very recently, Dr Ed Carter (above), based at the Barts Cancer Institute in London, and funded by Breast Cancer Now, developed a method to recreate Ductal Carcinoma In Situ (DCIS) in the lab, using human cells donated by patients to the Breast Cancer Now Tissue Bank.
DCIS is an early tumour which is contained within the ducts of the mammary gland. In up to 50% of cases, DCIS will progress into full invasive breast cancer, and for the rest it may pose no harm. But it’s very difficult to predict whether or not a woman’s DCIS will be the one that progresses. Because of this, everyone with DCIS, roughly 6,700 women a year, is offered treatment, even though at least half of them may not need it.
Finding ways to accurately predict whether or not DCIS will progress is an important goal of Ed’s work. Recreating the duct structure in the lab allows him to understand the interplay between the ‘luminal’ cells which line the breast duct, and the outer ring of ‘myoepethelial’ cells which contain them. It is this layer of myoepithelial cells which surrounds the malignant cells in DCIS, but which breaks down if DCIS becomes invasive cancer.
Using cells donated to the Tissue Bank gives Ed more certainty that his DCIS ‘model’ represents how DCIS really behaves in patients.
He explains how it works: “We’re able to access and isolate pure populations of normal luminal and myoepithelial cells, grow those in the lab, modify them how we want, and then put them back to together – wherein they’re able to reform into a native duct structure.”

Manipulating the genes in either the luminal or myoepithelial cells causes the ‘progression’ of the model, from the normal breast duct structure, to DCIS, through to invasive breast cancer. And the results never fail to amaze Ed.
“Every time I see the model form and replicate these different stages, it reminds me of the reason why I got into science. At each stage of this we’re never quite sure whether it’s going to work, but it does – the bilayer structure breaks down, the cells break away from their initial confines, and they do it robustly – it’s quite exciting to see.”
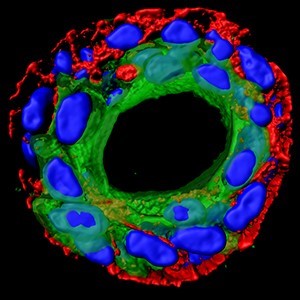
Ed and his colleagues are looking to use their model to answer some critical questions about the progression of DCIS to invasive breast cancer, including how myoepithelial and luminal cells communicate. They hope this will lead to better ways to predict whether or not a person’s DCIS will progress, and develop new treatments to help prevent this.
We’re grateful to the Haley Family Trust for their generous support of Dr Ed Carter’s work. The initial work was funded by Rosetrees Trust and Barts Charity.
Dr Rachael Natrajan – screening for genes
By reading the DNA from breast tumours donated by patients, scientists have identified hundreds of genes that are repeatedly altered or mutated in breast cancer. This suggests that these genes may be involved in cancer – but for many of these genes, their exact role is still unclear.
However, to study each one of hundreds of genes individually to find out how it’s involved in breast cancer is a huge task, and to carry on using precious samples donated by patients could be potentially wasteful.
Breast cancer cells grown in the lab, known as ‘cell lines’, are well suited to this challenge for a couple of reasons. One is the ability to scale up experiments, as you can keep on growing as many cancer cells as you need. This provides an almost limitless supply of opportunities to test your ideas, whether you’re testing one gene or one hundred genes.
Another advantage is consistency. Since the starting material is the same, all of those hundred experiments should be exactly the same, the only difference being the genes your studying, giving more confidence that the results can be relied upon.
One researcher using cell lines to do experiments on an almost industrial scale is Dr Rachael Natrajan, who is trying to understand which genes drive growth in different types of breast cancer. To do this, her team, from the Breast Cancer Now Toby Robins Research Centre at the Institute of Cancer Research in London are testing hundreds of ‘mini-tumours’, in something akin to a factory production line.
Breast cancer cells are formed into small balls, each one contained in a well, 96 of them crammed on a plate smaller than a postcard. Then, a special process silences the activity of genes – one gene per cell ball – before letting the whole thing mature for a few days in an incubator. Finally, high-tech machinery captures an image of each individual ball to measure the growth rate of these mini-tumours.
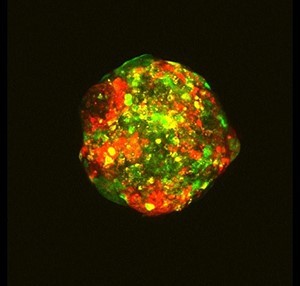
It’s a crucial detail that their mini-tumours are 3D, rather than cells grown in 2D on a flat plastic Petri dish. This is critical to understanding breast cancer better – it’s not just that breast tumours in patients don’t grow in flat sheets, the 3D nature of a tumour can actually make it more aggressive.
Many tumours grow so fast that the cells in the centre don’t have an adequate blood supply to provide them with the oxygen and nutrients they need. But instead of weakening the cancer cells, these difficult conditions mean the cancer cells face a ‘fight to survive’, making them tougher to kill and more likely to spread.
Dr Barrie Peck from Rachael’s team likens growing cells in 2D to “growing plants in greenhouses – they have all the water, food, and sunlight they could possibly need, they’re grown in perfectly optimal conditions. But actually, the tumour environment is more harsh, like a storm-blown tree on a mountain top – and so to better understand cancer we have to get closer to that. By putting the cancer cells in this more competitive environment, we can really test their capacity to grow, survive, and spread.”
Using this technique, genes which affect the growth and survival of these mini-tumours can be identified on a large scale – hundreds of genes can be screened in multiple different types of breast cancer cell in one day. This means that they might spot things that others miss, as Barrie explains. “Rather than just focussing on your favourite gene, and asking the question whether that gene is involved in, say, tumour growth – instead you’re looking at 200 genes, and trying to figure out which are the most important. This matters, because your favourite gene might actually turn out to be number 100 on the list of the most important genes, but what you really want is the top five.”
The genes which they identify as being potentially important can then be further investigated using alternative methods. But by using their mini-tumours to whittle down the list of genes, there’s a much greater chance of success later on. And their work is already producing the goods. “We’re finding mutations that people didn’t think played a role in breast cancer before”, says Rachael, “and we’re demonstrating not only that they do, but that they’re actually pretty important, and we can even start to predict which drugs would better treat those specific cancers.”
Dr Rebecca Marlow – creating patient ‘avatars’
How close can cells in a lab get to representing tumours in real patients? One researcher trying to answer that question is Dr Rebecca Marlow, who shares her expertise between our Research Centre in South Kensington, London, and our King’s College London Research Unit, in Guy’s Hospital near London Bridge.

One method of studying a patient’s breast tumours is by taking small samples of them and implanting them into special breeds of mice that lack an immune system. This way, the tumours are not only kept alive, but they also stay remarkably like how they were in the patient. These are called patient derived xenograft (PDX) models.
Rebecca’s team also grows these patient cells in the lab as well, essentially growing real tumours outside of human (and mouse) bodies. These tumours – called ‘Patient Derived Organoids’ – can then be used to test drugs, with the hope being that they can reflect how the patients from whom they came, would respond to drugs in real life.
“We know that these PDX animal models are the gold standard in the field currently, when it comes to representations of patients’ tumours,” Rebecca explains. “We have some funding from Breast Cancer Now and the organisation NC3Rs to understand whether patient derived organoids could reduce or replace animal use. If you see the same response in the organoids and animals, why would you need to do the work in animals? That is the question we are currently investigating.”
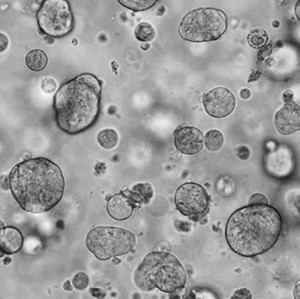
Growing samples of tumours donated by patients is challenging and requires a complex recipe of nutrients and scaffolding structures. But in theory, the pay-off is that these organoids could be the closest thing in the lab to represent how a real tumour grows in a patient.
Rebecca and her colleagues are currently testing these organoids to understand how close they really are to real tumours, starting with organoids developed from triple negative breast tumours, a hard-to-treat form of the disease. They’ll also be investigating new potential drug targets such as KIFC1, discovered earlier in the year at our King’s College London Research Unit.
There is even the possibility of using these organoids as ‘avatars’, using them to make decisions about the treatment given to the patient who donated the tumour in the first place, as Rebecca explains: “In the future we’d ultimately like to impact patient care in real-time with these organoids. Imagine that you could take a sample of tumour from a patient, derive an organoid from it, test a range of drugs, and then go onto inform which drugs that patient should have next – rather than the prescribed normal pathway of patient care. We’re not there yet but that’s ultimately where we’d like to be.”
Our scientists are using many different methods to study breast cancer, which includes research involving cells, animals, patient samples, and people with breast cancer participating in studies and helping researchers answer important questions. The exact methods of research will depend on the question at hand, and is always backed up by the best evidence, rigorously assessed by a panel of experts.
The coronavirus pandemic has put a lot of our lab-based research on hold. But we don’t want to lose momentum or to let progress stall. We need your support, now more than ever, so that our researchers can make up for lost time.
These three projects are just a snapshot of what we have going on at Breast Cancer Now, and all of our work is focused on one aim: to ensure that everyone who develops breast cancer will live, and live well.