In some exciting new research, Breast Cancer Now scientists led by Professor Chris Lord at the Breast Cancer Now Toby Robins Research Centre in the Institute of Cancer Research in London have uncovered a key weakness which could bring new hope for people with lobular breast cancer.
What’s more, they found that this weakness can be exploited through something called ‘synthetic lethality’. Sound scary? It is to tumours, as this weakness is proving to be an efficient way to eliminate specific cancer cells without harming healthy cells. But what is this ‘lethal weapon’ and how does it work?
What is synthetic lethality?
Our cells have thousands of different genes coding for proteins that work together to keep cells healthy. When one or more of these genes stop working properly it doesn’t have to mean the cell can no longer survive; there are often other proteins that can step in as a backup. In fact, in cancer our genes are often damaged, dysfunctional or lost altogether, but instead of dying the cells grow and spread far more than they should.
While faulty genes can give cancer cells a survival advantage, these mutations also make cells vulnerable to further damage. When two genes have a ‘synthetic lethal’ interaction it means that the simultaneous loss of both of them will cause the cell to die. If just one is lost or damaged, the cell can stay alive, but as soon as the other gene in the pair is broken or blocked, the cell is no longer able to survive. When either gene is removed, the pathways that rely on them are disrupted, and when both are removed at once, there is no longer a backup to step in.
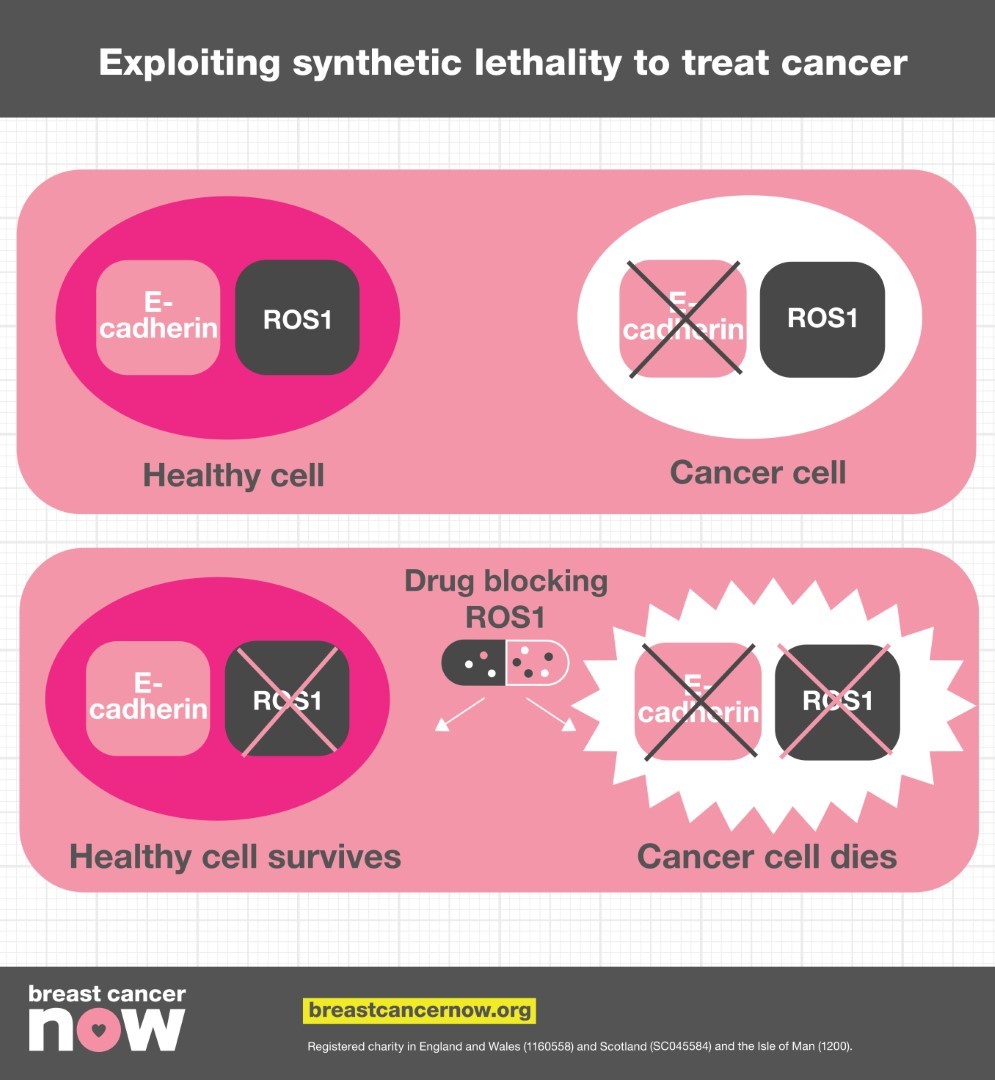
Synthetic lethality therefore presents opportunities to develop new cancer treatments. If a gene that’s often damaged in certain tumour cells has a synthetic lethal partner gene, creating a drug that blocks the actions of the other gene will destroy the cancer cell, without killing healthy cells.
PARP inhibitor pioneers
Breast Cancer Now scientists are not only breaking new ground with synthetic lethal cancer drugs today, but have actually been part of the story from the very beginning. The first breast cancer drug to use synthetic lethality to kill tumour cells was olaparib – a PARP inhibitor. Back in 2005, a team of scientists led by Professor Alan Ashworth at the Breast Cancer Now Research Centre contributed to the initial discovery that the 5% of breast cancers arising in women with an inherited BRCA1 or BRCA2 gene mutation had a synthetic lethal interaction with the PARP gene. Both BRCA and PARP are responsible for repairing damaged DNA, and the team realised that in BRCA mutated cancers, blocking the PARP protein caused the cells to die. Initially used as a treatment for BRCA mutated ovarian cancers, we heard the promising news at the start of 2018 that olaparib had been licensed by the US FDA to treat BRCA-mutated breast cancers that had spread to other parts of the body.
A key member of that team back in 2005 was Professor Chris Lord, who is not only continuing to push forward our knowledge of PARP inhibitors, but has now revealed a brand new synthetic lethal gene pair in lobular breast cancers.
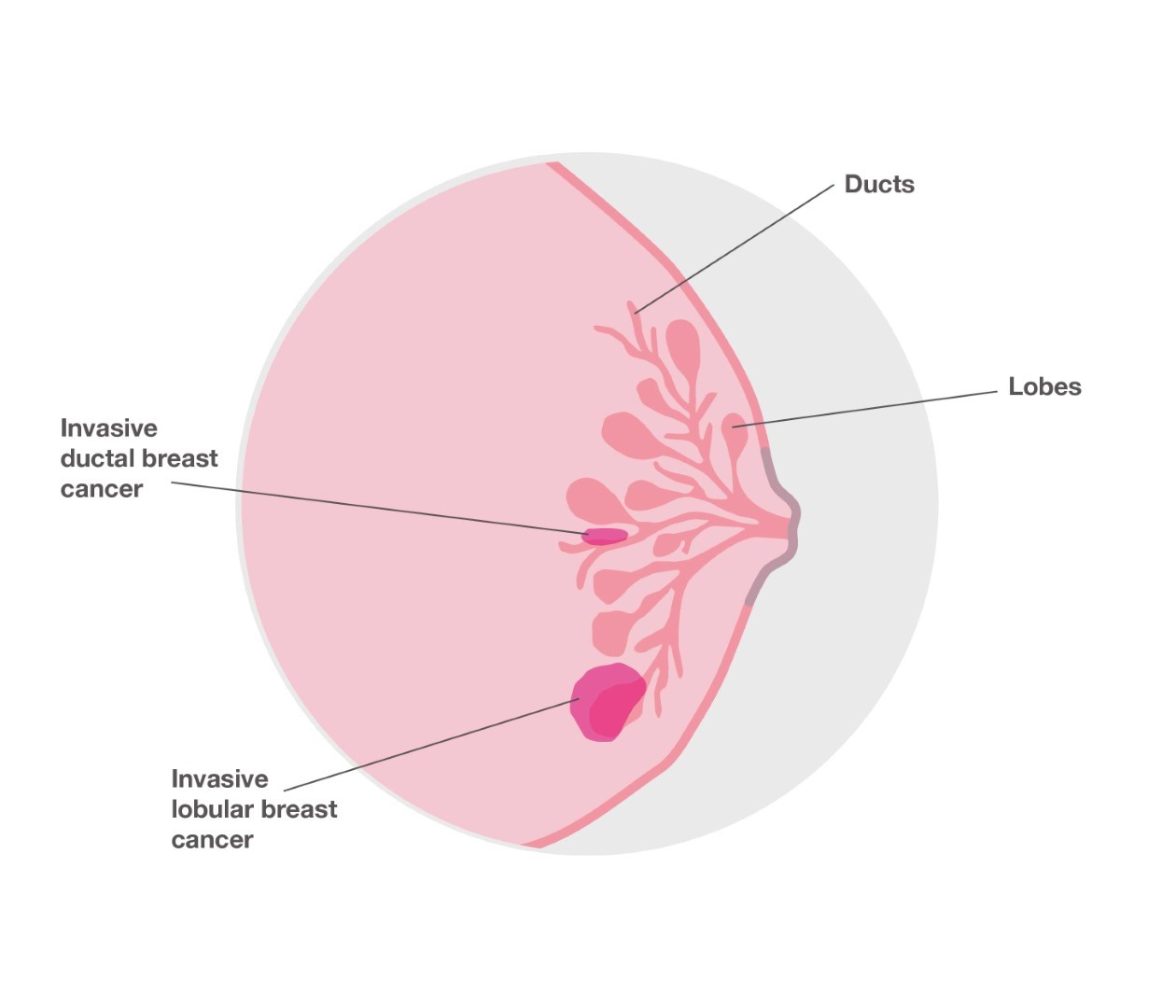
What is lobular breast cancer?
Most cases of breast cancer start in tubes called ducts that carry milk to the nipple. But around one in 10 breast cancers start in the glands where milk is produced. This is called lobular breast cancer. Lobular breast cancer tends to grow differently to other types of breast cancer, which means it is less likely to form a lump and more likely to produce a thickening or hardening of part of the breast. It can also be difficult to spot lobular breast cancer on a mammogram. Despite these differences, current treatment for lobular breast cancer is similar to other types of breast cancer and involves a combination of surgery, radiotherapy, chemotherapy, hormone therapy and/or targeted therapy.
Unfortunately, lobular breast cancers don’t always respond to chemotherapy as well as other breast cancers, and some forms are also less responsive to hormone therapy. For this reason, it is important that we find new and more effective treatments to give these patients the best chances of survival.
A new synthetic lethal weapon
The protein E-cadherin is responsible for holding cells together in the right place, acting like a cellular ‘Velcro’. Changes in the E-cadherin gene are one of the most frequent alterations in human cancer, with 13% of breast cancers and 90% of lobular breast cancers containing E-cadherin mutations. Despite how common alterations in this protein are, there are currently no treatments that specifically target this weakness in breast tumours, because little was understood about how this defect affects the tumour. Professor Lord wanted to find out more about cells with E-cadherin defects, and see if he could find a synthetic lethal gene partner that could form the basis of an efficient new treatment.
His team therefore tested 80 drugs in the lab to see if any of them led to the death of E-cadherin deficient breast cancer cells – and they were successful. They found that a drug blocking the protein ROS1 – a type of cell surface receptor - killed these cells. The team confirmed these results in mouse models of lobular breast cancer, and also found that the drug could kill tumour cells that had become resistant to anti-hormone therapy.
Creating brand new drugs can take a long time, but luckily drugs that block ROS1 already exist; crizotinib is a drug currently used to treat advanced lung cancers, and could be a potential new treatment for lobular breast cancers that have an E-cadherin mutation.
Looking to the future
This new discovery is so promising that Breast Cancer Now scientists will be carrying out a clinical trial to test the effectiveness of crizotinib in patients with E-cadherin defective, ER positive, advanced lobular breast cancer. The ROLO trial, funded by the Breast Cancer Now Catalyst Programme in collaboration with Pfizer, is the only recruiting study worldwide investigating new therapies specifically for advanced lobular breast cancer patients. Professor Lord will also be continuing to build on his recent discovery in his own Breast Cancer Now Catalyst Programme research project, investigating the effects of crizotinib and another ROS1-blocking experimental drug lorlatinib in breast cancer.
With E-cadherin defects being one of the most common genetic changes in all human cancers, treatment with ROS1-inhibitors such as crizotinib could potentially be useful in treating a number of other cancers, including stomach cancers.
Synthetic lethality is still a relatively untapped resource, but these new results could be paving the way for the first new synthetic lethal breast cancer drug since PARP inhibitors, and a new lethal weapon in our armoury against breast cancer.
The coronavirus pandemic has put a lot of our lab-based research on hold. But we don’t want to lose momentum or to let progress stall. We need your support, now more than ever, so that our researchers can make up for lost time.