From a lab in the Scottish city of Dundee, Breast Cancer Now Scientific Fellow Dr Jean-Christophe Bourdon is making new discoveries about a protein that has changed the face of cancer research.
This protein is sometimes known as the ‘Guardian of the Genome’ as its vital job is to protect our DNA from damage and our cells from becoming cancerous. Present in every cell in a person’s body, it takes on responsibility for an enormous number of tasks involved in the cell’s life, death, growth and repair.
The gene that makes this protein, however, is the most frequently mutated gene in most human cancers, including breast cancer, and could be considered the most widely studied aspect of the disease.
Dr Bourdon is working today at the forefront of research into this protein, called p53, but its story actually begins 39 years ago, in 1979…
A mystery protein emerges
Flashback to the end of the 1970s, to the year the Sony Walkman hit the shops and ‘Alien’ graced our cinemas. Not a great deal was known about cancer but scientists had discovered that certain cancers could be caused by viruses. Because of this, many were investigating one virus in particular, called SV40, which was known to cause cancer in animals. Scientists were intrigued by an unknown protein the virus made that they believed could be responsible for turning healthy cells into cancer cells.
Their investigations however revealed some unexpected results. The protein was in fact not made by the virus, but by the animal itself, making it a brand new, previously unknown protein produced by animals and humans that was somehow involved in cancer. Named p53 because it weighed 53 kilodaltons, this new protein was exciting news in the cancer world. After its discovery was announced by a few different teams of scientists worldwide, it drew the attention of many cancer researchers.
Putting together new pieces of the puzzle
Lots of scientists started to investigate this exciting new protein to discover more about its link to cancer. Many believed the gene that makes p53 – called TP53 – must be an ‘oncogene’. Oncogenes are genes with the potential to turn a normal cell into a cancer cell when they become mutated. As more research was done, it became clear that this was not the case, as TP53 was lacking in the characteristics needed to qualify as an oncogene. No one seemed to be able to pin down exactly what the function of TP53 was, so the once exciting protein started to fall out of favour.
In the 10 years that followed, technology and knowledge continued to grow and improve, meaning scientists could study and understand cancer in a way they never had before. Thanks to these improvements, in 1989 scientists were finally able to solve the mystery of p53’s identity - its gene, TP53, was found to be a ‘tumour suppressor’. Tumour suppressors are a group of protective genes. They make proteins that can stop healthy cells from becoming cancerous by controlling how cells grow and develop. Tumour suppressor genes had only recently been discovered and TP53 became the second ever described.
Scientists now had another vital piece in the p53 puzzle, and our mystery protein became a hot topic in cancer research once again.
Scotland steps into the spotlight
Our story continues in 1990s Dundee, where one of the original discoverers of p53 - Professor Sir David Lane - decided to create a new home for p53 research. In that same lab in 2005, Dr Jean-Christophe Bourdon made a discovery that changed the way we think about this important protein, and we have been supporting his work ever since.
 Professor Sir David Lane (Left) - one of p53’s original discoverers - with Dr Bourdon (Right)
Professor Sir David Lane (Left) - one of p53’s original discoverers - with Dr Bourdon (Right)
Dr Bourdon revealed that p53 isn’t just one protein. Its gene actually makes 12 different versions of the p53 protein that are all very similar, but have small important differences in how they look and act.
Same, but different…
It is quite common in our bodies for certain genes to make multiple versions of their proteins, and these are known as isoforms.
Isoforms occur because genes are made up of different sections. Sometimes the protein-making machinery inside our cells will read all of these sections and turn them into a protein, and sometimes they will choose to miss some out. These proteins are still created from DNA from the same gene, they just look or act slightly differently from one another.
Think of this as similar to a recipe in a cookbook: if you’re making a burger you can read the whole recipe, and put every ingredient in – including the cheese, the lettuce, and the tomatoes. But sometimes you might only want to add the lettuce though, or maybe you just want the meat and bun. Despite the variations, the meals you’ve made are all still burgers, they’ve come from the same recipe, but they all taste and appear slightly different.
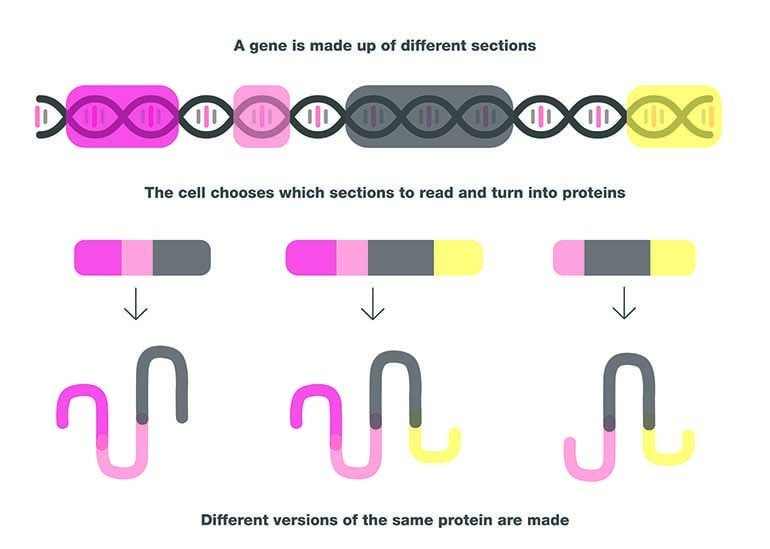 When different sections of the same gene are read by a cell’s protein-making machinery, the results are isoforms – slightly different versions of the same protein.
When different sections of the same gene are read by a cell’s protein-making machinery, the results are isoforms – slightly different versions of the same protein.
What does all this mean for breast cancer?
The tumour suppressor gene TP53 is supposed to be protecting our cells from cancer, but sometimes people can have a particularly aggressive or hard to treat cancer, despite having a normal version of the gene. Alternatively, some breast cancers respond very well to treatment, even though they might have a faulty TP53 gene. This had been puzzling cancer scientists, until a recent finding by Dr Bourdon and his team offered an explanation.
They found that one version of p53 – with the catchy name Δ133p53β – was more common in breast cancers that recur or spread. This p53-beta, as we’ll refer to it, is involved in the ability of cells to stick together or move around, and the breast cancer cells that contained lots of p53-beta were more likely to break away from the original tumour and spread to new places. This seemed to happen regardless of whether p53-beta was being made by a normal or mutated TP53 gene.
This is big news – it means that p53, the ‘guardian of the genome’, isn’t always protecting us from cancer, and some forms of the protein could be making things worse. It could also explain why some treatments don’t work as well as we think they should, as p53-beta could be sabotaging the outcome.
It isn’t all bad news, though. Whilst p53-beta could make breast cancer more invasive, it could also be making some tumours much more susceptible to certain treatments. Since p53 has such a wide range of roles within the cell and is involved in so many processes, these isoforms could be influencing the response to many kinds of cancer drug.
 Dr Jean-Christophe Bourdon and his team in their lab at the University of Dundee.
Dr Jean-Christophe Bourdon and his team in their lab at the University of Dundee.
Finding the right treatments for the right people
Currently it is difficult to know which specific breast cancer treatments someone will respond best to, but based on the work done so far it appears some important answers could lie in these 12 isoforms of p53. Knowing how they influence drug response could help scientists to develop a test to find out how much of each p53 isoform a person’s breast tumour has, and choose the treatment that will work best for them.
Knowing how to use currently available treatments in the best way will help stop people dying from breast cancer, as well as sparing them from the side effects of drugs they don’t need, and giving them a better quality of life while living with the disease.
The end of the story?
There is still a lot left to find out about p53 and its role in cancer, but Dr Bourdon believes “We can see light at the end of the tunnel”. Since Dr Bourdon’s discovery of the p53 isoforms in 2005, he has built an international community of researchers working on the isoforms, which has led to the understanding that their impact could reach beyond breast cancer into many other diseases. To get to where we are today, Breast Cancer Now has invested around £1.3million in p53 research in Dundee alone since 2005, and a further £540,000 across the rest of the UK.
 Attendees of Dr Bourdon’s 2017 workshop on p53 isoforms in Bergen, Norway. In the centre is eminent breast cancer scientist, Professor Mina Bissel, to the left of her is Dr Bourdon, and to the right is Professor Sir David Lane.
Attendees of Dr Bourdon’s 2017 workshop on p53 isoforms in Bergen, Norway. In the centre is eminent breast cancer scientist, Professor Mina Bissel, to the left of her is Dr Bourdon, and to the right is Professor Sir David Lane.
Back in 1979 no-one knew just how important p53 would turn out to be, but this protein has since been mentioned in over 87,000 scientific papers and has gone on to change the entire field of cancer research. When scientists first make a discovery, it can be difficult to predict how big an impact that discovery will have. All of Breast Cancer Now’s scientists are working towards new discoveries every day bringing us closer to our goal that by 2050, 71 years after the discovery of p53, everyone who develops breast cancer will live.
We would like to thank Asda for their support, which made Dr Jean-Christophe Bourdon’s Breast Cancer Now Fellowship – starting in 2012 – possible.
If you are interested in reading more about the story of p53, Dr Bourdon recommends Sue Armstrong’s book ‘p53: The Gene that Cracked the Cancer Code’.