The big questions
Sometimes referred to as ‘basic research’, the early stages of research are all about curiosity, exploring, and trying out new techniques.
This early science focuses on finding out the background information first, and identifying which areas need deeper exploration. It’s driven by the big questions like 'how do healthy cells become cancer?', 'why do people get breast cancer?' or 'what is happening inside a cancer cell?'.
Once scientists start to explore these topics, they begin to uncover more specific questions that guide the direction their research takes.
Exploring deeper
If, for example, an early study has found that cancer cells have higher levels of a certain protein than healthy cells, scientists will now have new questions to explore. What is this protein’s role in the cell? Does it help the cancer to grow? What happens if we remove it?
Breast Cancer Now funds lots of research teams across the UK and Ireland who are performing these kinds of exploratory projects. Our scientists are asking questions like how do normal processes go wrong in the breast?, what are the characteristics of different types of breast cancer? or what makes breast cancer spread?
This initial research may be many years away from improving the way cancer is prevented, detected or treated, but it is all vital for providing the knowledge needed to take the science further. Without always knowing it, these initial investigations are the first steps towards finding new or better ways to treat diseases such as breast cancer.
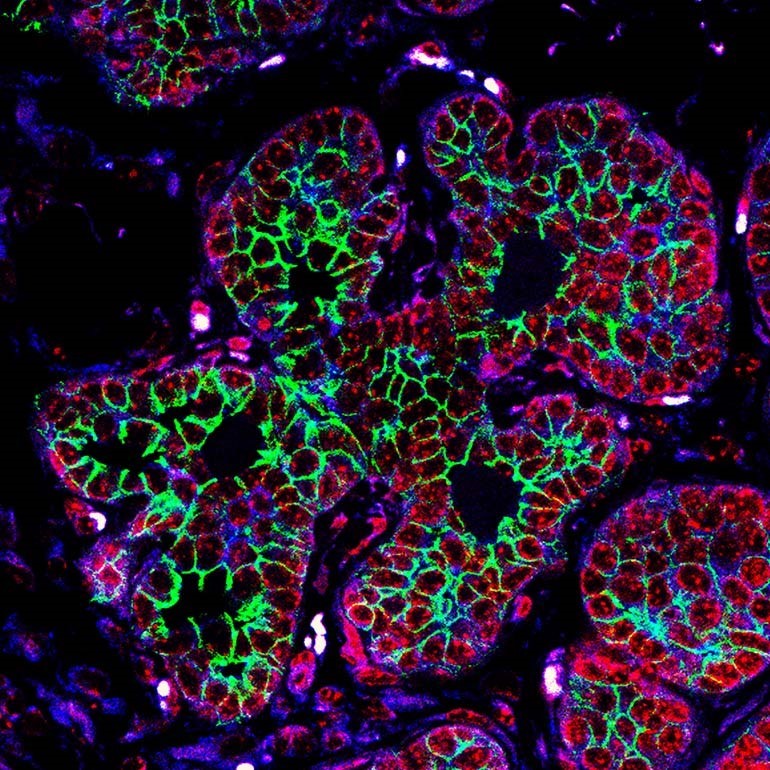 Imaging techniques are useful in early research to explore what is happening inside cancer cells. The green shown here is a protein involved in cell movement. The levels of this protein increase as the cancer cells become more invasive and mobile. Image: Dr Sarah Boyle.
Imaging techniques are useful in early research to explore what is happening inside cancer cells. The green shown here is a protein involved in cell movement. The levels of this protein increase as the cancer cells become more invasive and mobile. Image: Dr Sarah Boyle.
How could this finding benefit patients?
Once basic research topics have been explored in greater depth, their potential to benefit patients starts to become much clearer. Finding out how to take this knowledge and use it to improve the lives of people living with disease falls under the umbrella of ‘translational research’. Often referred to as ‘bench to bedside’, this is the research that provides a bridge between the discoveries made at the lab bench, and real-world benefit to patients in the clinic.
To begin developing new drugs for patients, scientists first need to decide which part of the cell will make the best target. Is this the protein they found in large amounts, or maybe one that works alongside it? They then need to find or design a chemical compound – or a biological molecule such as an antibody - that not only fits the target, but also has the desired effect – be that turning the target off, or maybe activating it. Scientists might use computer programmes to carry out this design process, or they could treat cancer cells with a range of compounds to see which ones work best. The researchers might then need to refine their chosen compound, to make it as effective and safe as it can be.
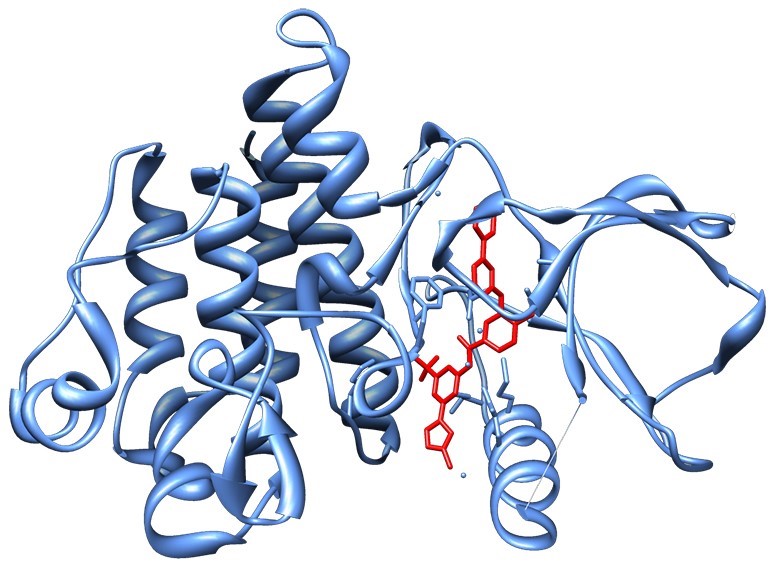 Computer models are a useful tool for researchers to work out whether a chemical compound (red) might fit a target protein (blue). Image: SocratesJedi [CC BY-SA 3.0], from Wikimedia Commons
Computer models are a useful tool for researchers to work out whether a chemical compound (red) might fit a target protein (blue). Image: SocratesJedi [CC BY-SA 3.0], from Wikimedia Commons
How does this new drug affect the whole body?
Testing new drugs on cells gives scientists useful information, but they also need to know how the drugs will affect the whole body. This is where animal models are needed, as they provide a wealth of information about how well the drug reaches a tumour, whether it stops the tumour from growing, what effect it has on the body, and if anything unexpected happens. By law all new drugs must first be tested on animals, but the use of animals in research is not an issue that Breast Cancer Now or our scientists take lightly. We are constantly striving to develop techniques that mean the use of fewer animals in research, and we only fund animal research if it is essential and there is no alternative.
Does this drug work in patients?
Now that the scientists have a drug and their research suggests it could work, they need to do clinical trials in patients to make sure, and answer some final, important questions.
Clinical trials are split up into phases, with each phase seeking a different set of answers. The process is rigorous to make sure that new drugs are effective and come with as few side effects as possible. Only a small proportion of drugs that enter clinical trials show positive enough results to bring them into routine clinical practice, as unfortunately not all drugs work as well as the experiments carried out up until this stage might indicate.
Phase 1 clinical trials ask big and general questions to determine whether it is worth taking the drug further. Usually the drug is tested on a small number of people, to answer: “is it safe?”, “How much of the drug should we give?” and “what does it do to both the cancer and the rest of the body?”
If the drug is safe and works as expected it can enter a Phase 2 trial. This usually involves testing on a larger group of people and refines the previous questions, asking “how well does the drug work?”, “Who might it work best for?”, and “What are the side effects?”. The researchers may then find ways to reduce those side effects.
Phase 3 is where the ultimate decision happens. Does this drug work better than what we currently use? We don’t want to spend time developing drugs that may work better than nothing, but don’t work better than what patients are already taking. We want to make sure this new drug offers a better alternative to the current standard of treatment, be it more effective, or with fewer side effects. Phase 3 could also be used to find new ways to give existing treatments, for example whether a drug could be improved by giving it at a different dose or a different point in the treatment journey.
Sometimes in the earlier stages of research, scientists might discover that a drug already used to treat other conditions could work on their target. Since we already know this drug is safe to give to people, the process is sped up and it can enter the later stages of the clinical trials much quicker than a brand-new treatment.
After all these questions have been answered, if we are left with a drug that works and is safe the manufacturer can apply for a licence for it. This involves providing information from all the experiments to the regulatory agency (currently the European Medicines Agency) who review it and decide if the drug is effective and safe enough to be marketed for the specific disease or condition. All the important information about how this drug works in those patients, and any side effects that may occur can then be provided with the drug.
There are situations where doctors might prescribe a drug that isn’t licensed for a patient’s specific condition (this is known as ‘off-label prescribing’) where research shows that it would help. In England, after a drug has been licensed the National Institute for Health and Care Excellence (NICE) will normally decide whether the drug should be routinely available to patients being treated by the NHS. NICE decisions are normally followed in Wales and Northern Ireland. In Scotland, the Scottish Medicines Consortium (SMC) makes these decisions.
The questions don’t stop there
You may think that once a drug is licensed and being used to treat patients that the testing would finish, but research continues to make sure it’s working as well as it can.
A Phase 4 clinical trial seeks to discover the long term effects of the drugs, and pay attention to any new information that wasn’t known before. We also want to make sure people are taking the drugs that are most likely to work against their specific cancer, and that they are able to cope with side effects of their treatments. Our scientists are investigating which drugs might work best for which breast cancer patients, whether we can predict if a person’s cancer might become resistant to a drug, and how to make sure people living with breast cancer have the best quality of life possible.
The journey from initial explorations in the lab to new ways to treat breast cancer takes time, sometimes many decades, but high quality research can speed the process up. In 2005, Breast Cancer Now scientists discovered that blocking a protein called PARP could kill breast and ovarian cancer cells with BRCA mutations. 13 years later, a drug called olaparib that does just this is now being used to treat ovarian cancer patients, and is in the process of reaching breast cancer patients in the UK too.
The coronavirus pandemic has put a lot of our lab-based research on hold. But we don’t want to lose momentum or to let progress stall. We need your support, now more than ever, so that our researchers can make up for lost time.